European Launch of ResistancePlus MG FleXible for the GeneXpert System
SYDNEY--(BUSINESS WIRE)--Dec 16, 2019--
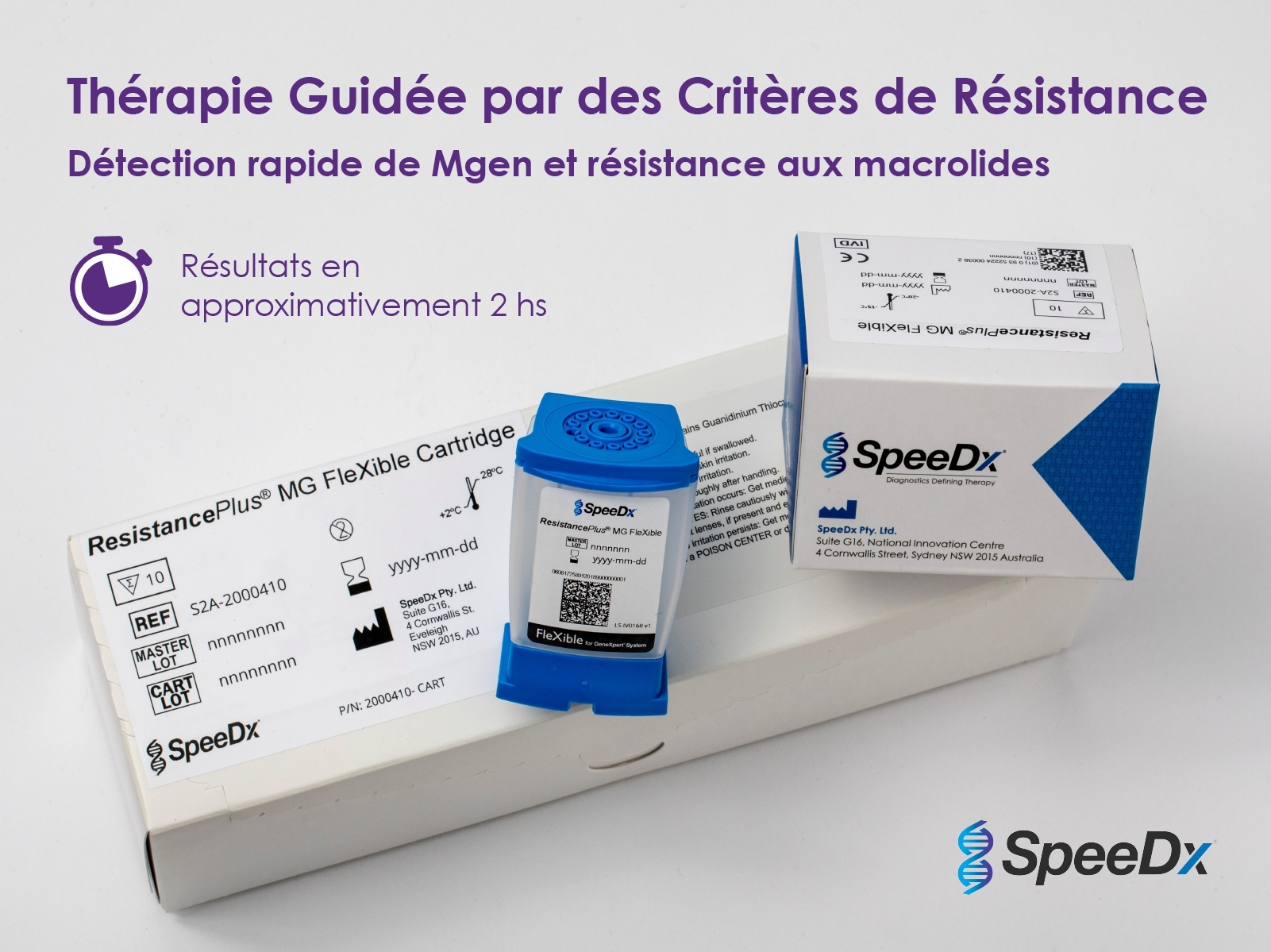
SpeeDx Pty, Ltd. today announced the European launch of ResistancePlus® MG FleXible, the first sample-to-answer test for detecting the sexually transmitted infection (STI) Mycoplasma genitalium (Mgen) and markers associated with azithromycin resistance. Cepheid is the exclusive distribution partner of this test, available through Cepheid’s FleXible Cartridge programme for the GeneXpert System.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191215005039/en/
ResistancePlus MG FleXible is now selling in France and the U.K. (to be followed by rest of EU, Australia and New Zealand). This successful collaboration between SpeeDx and Cepheid is the first sample-to-answer test for Mgen and macrolide resistance - exclusively distributed by Cepheid. (Photo: Business Wire)
Management guidelines around the world, including those recently updated in France 1 and the U.K. 2, reference testing the macrolide resistance status of Mgen infections in order to more effectively treat patients. Mgen can cause symptoms such as urethritis, cervicitis, endometritis and pelvic inflammatory disease, and has been linked to reproductive health complications including pre-term birth and infertility. 2,3 Due to the challenges of rising antimicrobial resistance and a lack of clear data that asymptomatic infection leads to protracted health complications, Mgen testing guidelines are unanimous in their recommendation to only test symptomatic individuals.
“This is an important test expansion for the thousands of GeneXpert Systems on the market,” said Colin Denver, SpeeDx CEO. “ ResistancePlus MG FleXible enables Resistance Guided Therapy, supporting more clinicians to adhere to testing guidelines and maximising effective care for their patients infected with Mgen.”
Both SpeeDx and Cepheid will be showcasing ResistancePlus MG at RICAI (Réunion Interdisciplinaire de Chimiothérapie Anti-Infectieuse) December 15 th and 16 th in Paris. Visit SpeeDx booth #41bis and Cepheid booth #18 to learn more.
About SpeeDx
SpeeDx has developed a molecular diagnostics test portfolio, principally for infectious diseases, that provides both identification as well as therapeutic guidance capabilities. The company is based in Australia with offices in Austin and London, and distributors across Europe. SpeeDx specializes in molecular diagnostic solutions that go beyond simple detection to offer comprehensive information for improved patient management. The SpeeDx ResistancePlus ® MG open-platform assay is CE marked for sale in Europe, TGA approved for sale in Australia and clinical trials are being finalised for submission to the U.S. FDA. Products in the SpeeDx portfolio focus on multiplex diagnostics for sexually transmitted infection (STI), antibiotic resistance markers, and respiratory disease.
For more information on SpeeDx please see: https://plexpcr.com
- https://www.sfdermato.org/site/groupe-infectiologie-dermatologique-et-infections-sexuellement-transmissibles.html
- https://www.bashhguidelines.org/media/1146/ngu-update-05_2017-final.pdf
- http://www.sti.guidelines.org.au/sexually-transmissible-infections/mycoplasma-genitalium
View source version on businesswire.com:https://www.businesswire.com/news/home/20191215005039/en/
CONTACT: SpeeDx:
Europe, Australia, New Zealand:
Madeline O’Donoghue
+61 2 9209 4170
Darwa Peterson
Tel. + 1.408.400.4324
KEYWORD: AUSTRALIA/OCEANIA EUROPE AUSTRALIA FRANCE
INDUSTRY KEYWORD: BIOTECHNOLOGY HEALTH MEDICAL SUPPLIES MEDICAL DEVICES
SOURCE: SpeeDx
Copyright Business Wire 2019.
PUB: 12/16/2019 02:00 AM/DISC: 12/16/2019 02:01 AM
http://www.businesswire.com/news/home/20191215005039/en